New psoriasis drug anticipated to gain FDA approval
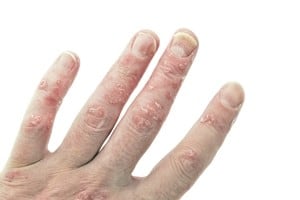 In pharmacy technology news, the Committee for Medicinal Products for Human Use (CHMP) – the European Medical Agency’s advisory council – has just announced its support of Novartis’ new drug secukinumab, a treatment for moderate-to-severe plaque psoriasis.
In pharmacy technology news, the Committee for Medicinal Products for Human Use (CHMP) – the European Medical Agency’s advisory council – has just announced its support of Novartis’ new drug secukinumab, a treatment for moderate-to-severe plaque psoriasis.
The drug, which will be marketed in Europe as Cosentyx, received a similar stamp of approval from U.S. Food & Drug Administration’s (FDA) Advisory Committee last month.
In general, recommendations from these committees result in approval of the drug by each corresponding deciding body. In this case, the European Commission will likely grant approval based on the CHMP’s recommendation, and the FDA is expected to approve the drug in early 2015.
According to FierceBiotech, if Cosentyx is approved, Novartis’ IL-17 blocker injection will be the first to go to market, and could potentially lead to a new standard of treatment options for psoriasis, psoriatic arthritis and other inflammatory diseases.1 Injection therapies are currently the most common option for those suffering from psoriasis.
However, managing symptoms can be a challenge and many treatments have left psoriasis patients generally unsatisfied. With psoriasis affecting 7.5 million Americans, the injection therapy could stand to better the lives of a large population.2
Road to approval
Novartis announced unanimous approval of the drug from the FDA Advisory Committee on Oct. 20, 2014. The phase III clinical program showed significant promise for using secukinumab as a psoriasis treatment, and current data highlighted no major safety issues associated with the drug.2 In the trial, 70 percent of patients achieved clear skin and 90 percent almost clear skin. Vas Narasimhan, global head development at Novartis Pharmaceuticals, explains:
“Today’s recommendation is based on the efficacy and safety data put forth in our robust clinical trial program and brings us one step closer to delivering an innovative, new treatment option for people suffering from moderate-to-severe psoriasis. We look forward to working with the FDA as it finalizes its review.”2
When the pharmaceuticals company announced on Nov. 21, 2014, that the CHMP had made an overwhelming recommendation, representatives of Novartis expressed similar excitement. Division head at Novartis Pharmaceuticals, David Epstein stated:
“With this exciting news, we may change the way psoriasis is treated, as 50 percent of patients are unhappy with their current psoriasis therapies, demonstrating an urgent need for new treatments that clear skin faster and for a longer time.”3
Cosentyx is already being called a better treatment for psoriasis than Enbrel, another treatment option that generates $4.6 billion in revenue annually, according to Bidness Etc.4 Until Cosentyx is officially approved and released to market, it is impossible to determine the exact effect the drug will have on the pharmaceuticals industry. Bloomberg predicts that Cosentyx may garner Novartis $1.1 billion in sales in the year 2020.5
Other Novartis news
Novartis may have another multi-billion dollar treatment option on the horizon. The unnamed drug is a treatment option for those suffering from chronic heart failure, and thus so far has impressed professionals across the health care industry. According to Reuters, Novartis plans to file an application for approval with the FDA in 2014 and in Europe next year.6 Though the timeline for this drug is still unclear, if approved, it could ultimately help millions of people experiencing heart failure, as well as lower the overall cost of patient care.
Such advancements show a lot of promise not only for Novartis Pharmaceuticals, but also for the health care industry on a broader spectrum. Providing the highest quality patient care is a commitment made by all health care professionals, and advanced, innovative treatments are increasingly making it possible to provide a higher standard of patient care.
1 “Novartis’ Enbrel beater bounds toward psoriasis approval ahead of the crowd,” by Damian Garde, Fierce Biotech, Nov. 21, 2014. http://www.fiercebiotech.com/story/novartis-enbrel-beater-bounds-toward-psoriasis-approval-ahead-crowd/2014-11-21
2 “Novartis announces FDA Advisory Committee unanimously recommends approval of AIN457 (secukinumab) for patients with moderate-to-severe plaque psoriasis,” Media Release, Oct. 20, 2014. http://www.novartis.com/newsroom/media-releases/en/2014/1864296.shtml
3 “Novartis Cosentyx(TM) receives positive CHMP opinion for first-line treatment of moderate-to-severe psoriasis patients,” Media Release, Nov. 21, 2014. http://www.novartis.com/newsroom/media-releases/en/2014/1873177.shtml#top
4 “Novartis (NVS) Receives CHMP’s Approval For Psoriasis Drug,” by Hannah Ishmael, Bidness Etc., Nov. 21, 2014. http://www.bidnessetc.com/29621-novartis-nvs-receives-chmps-approval-for-psoriasis-drug/2/
5 “Novartis Wins European Backing for Psoriasis Drug Cosentyx,” by Simeon Bennett, Bloomberg, Nov. 21, 2014. http://www.bloomberg.com/news/2014-11-21/novartis-wins-european-backing-for-psoriasis-drug-cosentyx.html
6 “Novartis heart failure drug provides host of benefits: study,” by Bill Berkrot, Reuters, Nov. 17, 2014. http://www.reuters.com/article/2014/11/17/us-health-heart-novartis-idUSKCN0J12AV20141117