FIND YOUR PATH
TO SUCCESS
With dozens of programs to choose from, there’s no better time than now to pave your path to your future.
Explore programs


Why Carrington?
REAL WORLD Skills
With many classes to guide you, you’re certain to be prepared for your career
Hands-on classes
Qualified instructors lead hands-on classes to give you real-world experience
Flexible learning options
Conveniently available online and at 16 of our locations
CAREER SERVICES
Career Services staff is focused on helping graduates find a job in their career field
Fast Track Learning
Carrington graduates earn their certificate of completion in as few as 7-15 months
Many Programs to
Choose From



Ready to Do This?
Find your path in the medical, dental, pharmaceutical, veterinary, or trades fields.

Rated 4.5 Stars on Google
Carrington students have thrived in so many different environments and in so many different professions. Many have gone on to work at some of the world’s highly respected companies.
We have had the privilege of interviewing countless happy students, many of whom have been kind enough to leave us glowing reviews about their experience.
Featured Programs

Medical Assisting
Carrington College’s Medical Assisting program prepares graduates to work in medical offices and clinics.
Learn More
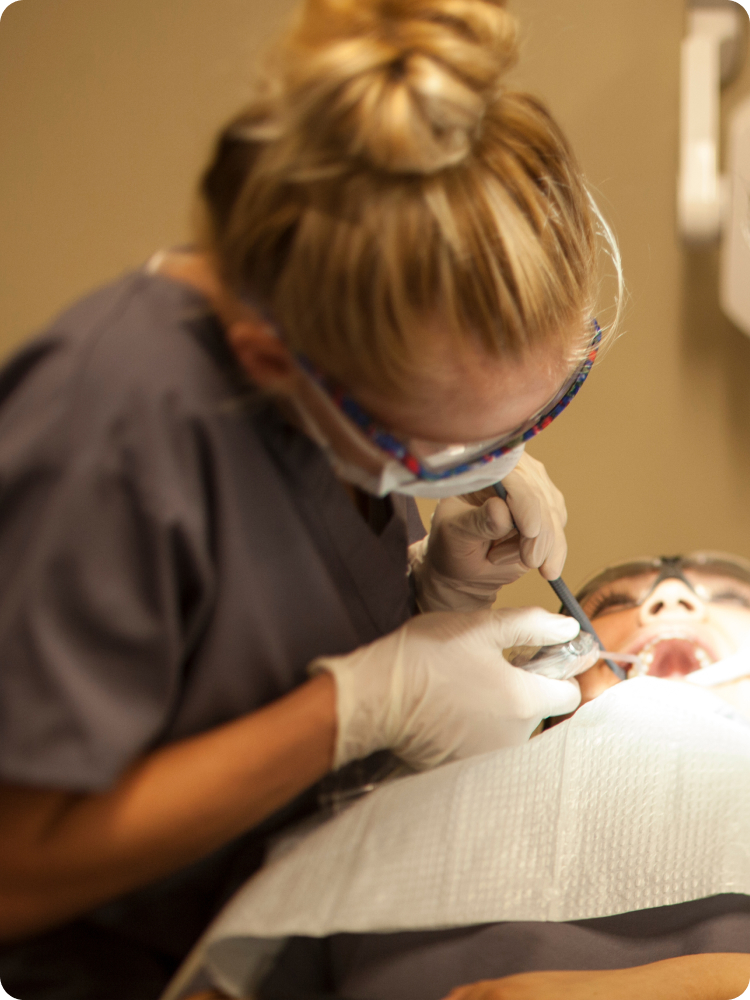
Dental Assisting
Carrington College’s Dental Assisting program trains students in all aspects of working with a dentist, including patient care, office, and laboratory duties.
Learn More

Pharmacy Technology
Prepare for an exciting career in health care with Carrington College’s Pharmacy Technology program.
Learn More

Veterinary Assisting
If you love caring for animals and working with customers, you may be a great candidate for Carrington College’s Veterinary Assisting program.
Learn More
Online and On-Campus
Programs are Open for Enrollment
Why Wait... Make the First Step to a Fresh Start Today!
Talk to an enrollment services representative about your options and earn your online certificate in as few as 7-15 months.

Upcoming Events
View our calendar to see what open houses and career
fairs we have scheduled.
fairs we have scheduled.
16 Locations
Carrington has 16 locations across the United States. If we don’t have a campus location that’s convenient for you, explore our online programs.
See Locations